Replicate Study Designs: Advanced Methods for Bioequivalence Assessment
 Feb, 3 2026
Feb, 3 2026
Why Standard Bioequivalence Studies Fail for Highly Variable Drugs
Imagine testing a drug that behaves wildly differently from one dose to the next in the same person. That’s the reality with highly variable drugs (HVDs)-medications like warfarin, levothyroxine, or certain antiepileptics where blood levels can swing by 40%, 50%, even 60% between doses in the same individual. Standard two-period crossover studies (TR, RT) were never built for this. They assume consistency. When that assumption breaks, you need more subjects-sometimes over 100-to get a reliable answer. That’s expensive, slow, and often impossible to recruit. The result? Failed studies, delayed generics, and patients stuck waiting for affordable options.
Enter replicate study designs. These aren’t just tweaks-they’re fundamental upgrades to how we measure bioequivalence when variability is the problem, not the exception. The FDA and EMA didn’t just allow these designs; they made them mandatory for HVDs. Why? Because without them, many life-saving generics would never reach the market.
How Replicate Designs Work: Three Main Types
Replicate designs aren’t one-size-fits-all. They come in three forms, each with specific strengths and regulatory requirements.
- Full replicate (four-period): TRRT or RTRT sequences. Each subject gets both the test and reference drug twice. This lets you calculate within-subject variability for both products separately-CVwT and CVwR. The FDA requires this for narrow therapeutic index (NTI) drugs like warfarin because precision matters. If the test drug is too variable, you can’t approve it.
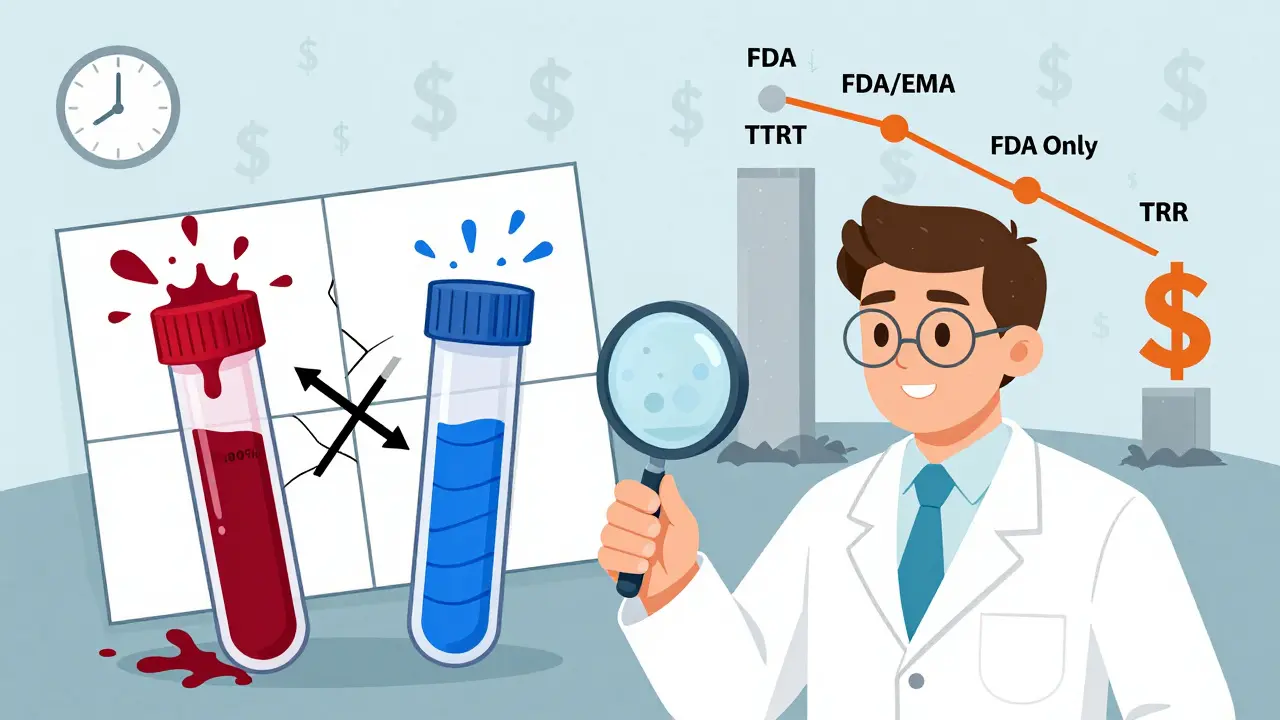
- Full replicate (three-period): TRT or RTR sequences. Subjects get the reference drug twice and the test drug once, or vice versa. This design estimates reference variability (CVwR) and is accepted by both FDA and EMA. It’s the sweet spot for most HVDs: fewer periods than four-period, but still enough to scale acceptance limits.
- Partial replicate: TRR, RTR, RRT. Only the reference drug is repeated. You can’t measure test variability, but you can still apply reference-scaling. The FDA accepts this for HVDs, but the EMA doesn’t. It’s cheaper and faster, but less informative.
For drugs with an intra-subject coefficient of variation (ISCV) under 30%, stick with the old 2x2 design. It’s simpler and just as good. But once you hit 30% or higher? Replicate designs become your only viable path.
Why Sample Sizes Drop by Half-And Why That Matters
Here’s the real game-changer: replicate designs cut the number of subjects you need by 40-70% for HVDs.
Take a drug with 50% ISCV and a 10% formulation difference. A standard 2x2 crossover would need 108 subjects to reach 80% power. A three-period full replicate? Just 28. That’s not a small savings-it’s the difference between a study that’s doable and one that’s financially impossible.
Why? Because replicate designs let you use reference-scaled average bioequivalence (RSABE). Instead of forcing all drugs to meet the same 80-125% acceptance range, RSABE widens the limits based on how variable the reference drug is. The more variable it is, the wider the window. But only if you can measure that variability accurately-and only replicate designs let you do that.
According to the FDA’s 2023 GDUFA report, 68% of HVD bioequivalence studies now use replicate designs. That’s up from 42% in 2018. And approval rates? 79% for replicate studies versus 52% for traditional ones. The data doesn’t lie: if you’re working with HVDs and you’re not using a replicate design, you’re fighting an uphill battle.

Regulatory Differences: FDA vs. EMA
The FDA and EMA agree on the need for replicate designs-but not on the details.
The FDA prefers full replicate designs, especially for NTI drugs. Their 2023 guidance on warfarin sodium mandates a four-period TRRT/RTRT design. They also accept partial replicates, but only if you use their specific statistical model. The EMA, on the other hand, leans heavily on the three-period full replicate (TRT/RTR). They don’t accept partial replicates at all. Why? Because they want to see both test and reference variability to ensure the test product isn’t more variable than the reference.
For global submissions, this creates a headache. A study designed for the FDA using a partial replicate might get rejected by the EMA. A four-period study approved by the FDA might be seen as overkill by the EMA. The International Council for Harmonisation (ICH) is working on a unified approach expected in late 2024, but for now, you need to tailor your design to the target market.
Bottom line: If you’re targeting the U.S., you have flexibility. If you’re targeting Europe, stick to the three-period full replicate. Don’t assume one design works everywhere.
Real-World Successes-and Costly Mistakes
There are plenty of wins. One CRO reported a levothyroxine study using a TRT/RTR design passed RSABE on the first submission with just 42 subjects. Previous attempts with 98 subjects using a 2x2 design had failed. That’s a 57% reduction in subjects and a year saved.
But there are also disasters. One statistician posted on a pharmacology forum about a four-period study for a long-half-life drug. They expected a 10% dropout rate. Got 30%. Had to extend recruitment by eight weeks and spend an extra $187,000. Why? They didn’t over-recruit. They didn’t plan for washout periods long enough. They assumed subjects would stick around.
Industry data shows dropout rates in multi-period studies average 15-25%. That means if you need 36 subjects, you recruit 45-50. Simple math. But many teams skip this step, thinking they’ll get lucky. They don’t.
Another common mistake? Using the wrong statistical model. You can’t just plug data into Excel. You need mixed-effects models, reference-scaling algorithms, and software like Phoenix WinNonlin or the R package replicateBE. The CRAN download logs show over 1,200 downloads of replicateBE in early 2024 alone. That’s not a niche tool-it’s the industry standard. If your team hasn’t trained on it, you’re behind.

Getting Started: What to Do Today
If you’re planning a bioequivalence study, here’s your action plan:
- Check the ISCV. If it’s under 30%, use a standard 2x2 crossover. If it’s 30-50%, go with a three-period full replicate (TRT/RTR). If it’s above 50%, use a four-period full replicate (TRRT/RTRT).
- Recruit extra subjects. Plan for 20-30% over-recruitment to account for dropouts. Don’t wait until you’re short.
- Choose your software. Train your analysts on replicateBE or WinNonlin. The learning curve is steep-expect 80-120 hours of training. Skip this, and you’ll get rejected.
- Align with the regulator. Know whether you’re targeting the FDA, EMA, or both. Don’t assume one design works everywhere.
- Don’t rush the washout. For drugs with long half-lives, make sure the washout period is at least five to seven half-lives. Otherwise, carryover effects will mess up your data.
And remember: if you’re working with a narrow therapeutic index drug, you have no choice. The FDA requires a four-period full replicate. There’s no workaround.
The Future: Adaptive Designs and AI
The next wave is adaptive designs. Imagine starting a study as a replicate, but if early data shows the drug isn’t as variable as expected, you switch to a simpler design mid-study. The FDA’s 2022 draft guidance allows this in specific cases. It’s still rare, but it’s coming.
Pfizer’s 2023 proof-of-concept study used machine learning to predict sample size needs based on historical BE data. It was 89% accurate. That’s not science fiction-it’s the future of study planning.
Market data confirms the trend: the global bioequivalence study market hit $2.8 billion in 2023, with replicate designs making up 35% of HVD assessments. That’s up from 18% in 2019. Companies like WuXi AppTec and PPD are investing heavily in this space because they know: the future belongs to those who can handle variability.
Final Takeaway: Replicate Designs Are No Longer Optional
If you’re developing a generic for a highly variable drug, you’re not choosing between a replicate design and a traditional one. You’re choosing between approval and rejection.
Regulators aren’t asking. They’re requiring. The data proves it: replicate designs work. They save time, money, and lives by getting safe, effective generics to patients faster.
But they’re not easy. They demand expertise, planning, and the right tools. The good news? The framework is clear. The path is defined. The only question left is whether you’re ready to follow it.
